Purpose-Built Particle Engineering
Technologies that enable bioavailability, stability, sensory performance, and manufacturability for complex actives.
Spray Drying
Closed-loop spray drying with precise control of particle size, morphology, and moisture for APIs and excipients.
Explore Spray DryingMicroencapsulation
Functional coatings and encapsulation for stability, taste masking, and targeted release of vitamins, minerals, and APIs.
Explore MicroencapsulationComplex Granulation
Wet and dry granulation engineered for flow, compressibility, and uniformity—ready for high-speed tableting.
Explore GranulationIron Deficiency Technologies
Gentle, bioavailable iron solutions with improved tolerability and sensory profiles for global nutrition programs.
Explore Iron TechFinished Dosage Forms
Tablets, capsules, blends, and ready-to-fill intermediates manufactured under cGMP with full QA oversight.
Explore Dosage FormsR&D & Tech Transfer
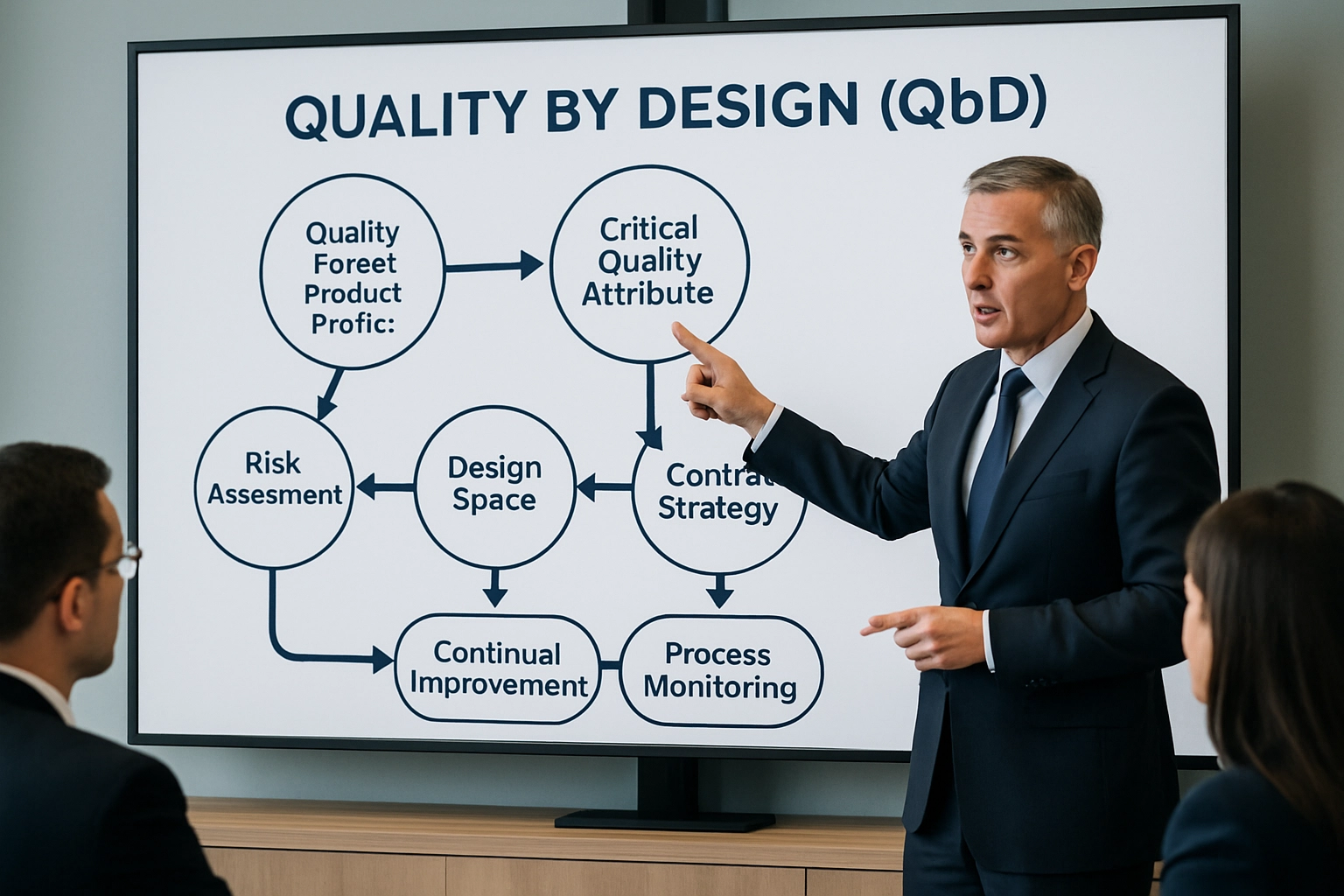
Quality by Design, DoE, and validation support that de-risk scale-up and global tech transfers.
Explore R&DFrom Feasibility to Commercial Supply
Multidisciplinary teams align formulation science, process engineering, and analytical controls to deliver right-first-time outcomes.
- Design of Experiments: Parameter optimization focused on CQAs and target performance
- Analytical Readiness: Method development, validation, and stability programs
- Tech Transfer: Structured protocols and documentation to ensure reproducibility
- Regulatory Support: cGMP batch records, traceability, and data integrity controls